Exploring the Precious Metals World: Heterogeneous Catalysts vs. Homogeneous Catalysts
Introduction: In the realm of catalysis, precious metals play a pivotal role in driving chemical transformations across various industries. Among the diverse catalyst types, heterogeneous and homogeneous catalysts stand out, each offering unique advantages and challenges. This article delves into the comparison between heterogeneous and homogeneous catalysts in the precious metals domain, shedding light on their characteristics, applications, and future prospects.
Heterogeneous Catalysts: Heterogeneous catalysts feature distinct phases between the catalyst and reactants, often with the catalyst being a solid while reactants are in the gas or liquid phase. Precious metals like platinum, palladium, and rhodium are commonly employed in heterogeneous catalysis due to their surface properties, which facilitate reactions without being consumed. These catalysts offer several benefits:
- Versatility: Heterogeneous catalysts find applications in numerous industries, including automotive, petrochemical, and pharmaceutical sectors, owing to their ability to catalyze various reactions.
- Stability: Precious metal catalysts exhibit high stability under harsh reaction conditions, ensuring prolonged catalytic activity and longevity.
- Recyclability: Unlike homogeneous catalysts, heterogeneous catalysts are easily separable from reaction mixtures, enabling their recovery and reuse, thus reducing operational costs and environmental impact.
Homogeneous Catalysts: Contrary to heterogeneous catalysts, homogeneous catalysts exist in the same phase as the reactants, typically in solution form. Precious metal complexes, such as organometallic compounds, serve as efficient homogeneous catalysts, offering distinct advantages:
- Selectivity: Homogeneous catalysts often demonstrate superior selectivity, enabling precise control over reaction pathways and product distributions, critical in fine chemical synthesis.
- Catalytic Activity: With homogeneous catalysts, the entire catalyst participates in the reaction, leading to higher turnover frequencies and potentially faster reaction rates compared to heterogeneous counterparts.
- Catalytic Mechanism Studies: Homogeneous catalysts facilitate detailed mechanistic studies due to their well-defined structures, aiding in the elucidation of reaction pathways and the design of improved catalysts.

Comparison and Future Perspectives: While both heterogeneous and homogeneous catalysts play crucial roles in the utilization of precious metals, each exhibits distinct characteristics suited for specific applications. Heterogeneous catalysts excel in industrial processes requiring robustness, recyclability, and ease of separation, while homogeneous catalysts offer unparalleled selectivity and mechanistic insights essential for complex synthesis routes.
Looking ahead, the integration of heterogeneous and homogeneous catalysis approaches, known as hybrid catalysis, holds promise in unlocking synergistic effects and overcoming individual limitations. Moreover, advancements in nanotechnology and catalyst design are driving the development of novel catalytic materials with enhanced activity, selectivity, and stability, paving the way for more sustainable and efficient chemical processes in the precious metals world.
Conclusion: In the dynamic landscape of catalysis, both heterogeneous and homogeneous catalysts featuring precious metals contribute significantly to industrial processes, from large-scale production to fine chemical synthesis. Understanding their distinct attributes and applications is paramount for harnessing their full potential in addressing current and future challenges in sustainable chemistry and catalytic technology.

Triple Diversification: Expanding Your Investment Strategy Beyond Borders and Banking
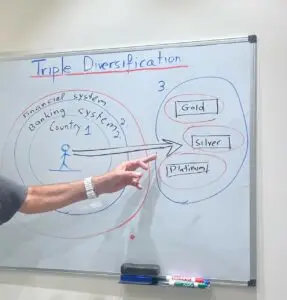
Triple Diversification: Expanding Your Investment Strategy Beyond Borders and Banking
Triple Diversification: Expanding Your Investment Strategy Beyond Borders and Banking In the realm of physical investments, diversifying one’s portfolio is often associated with the acquisition
Electrical Conductivity of Precious Metals
SummaryThis article explores the electrical conductivity of precious metals and their alloys, focusing on its measurement in Siemens per meter and variations across different materials.



