Atomic Absorption
Atomic absorption using ICP (Inductively Coupled Plasma) is a technique used to detect and quantify the presence of precious metals in various samples. The samples could be anything from soil to rocks, ores, or even jewelry.
In this technique, the sample is first dissolved in a liquid and then sprayed into an extremely hot plasma flame. The plasma is created by passing an electric current through a gas, which generates temperatures of over 10,000 degrees Celsius.
When the sample enters the plasma, the high temperature causes the atoms of the precious metals to become excited and jump to higher energy levels. As they return to their normal state, they release energy in the form of light at specific wavelengths. This light is then passed through a prism or a series of filters that isolate the wavelength of interest.
By measuring the intensity of this light at the specific wavelength, the amount of precious metal present in the sample can be determined. The ICP-AES technique can detect even trace amounts of precious metals, making it an extremely sensitive and accurate technique for assaying.
In simpler terms, Atomic absorption using ICP is like shining a very bright light on a sample to see what metals are present. The intensity of the light tells you how much of the precious metals are in the sample. This is useful for identifying and measuring the amount of precious metals such as gold and silver, in various materials, which can be used for a variety of purposes such as determining the value of jewelry, evaluating the quality of ores, or monitoring the levels of metals in soil and water.
The use of ICP in the precious metals industry has become increasingly important in recent years. Precious metals such as gold, silver, and platinum are highly valuable commodities and are used in a variety of applications, from jewelry to electronics. The purity of these metals is essential for their value, and ICP analysis can determine the precise amount of each metal present in a sample. In the precious metals industry, ICP is commonly used to analyze the composition of ore samples and to determine the purity of finished products. This information is used to establish the value of the metals and to ensure that they meet the quality standards required by customers. ICP analysis is also used to detect impurities in precious metals, which can affect their quality and value.
ICP-OES (Inductively Coupled Plasma Optical Emission Spectroscopy) and ICP-AES (Inductively Coupled Plasma Atomic Emission Spectroscopy) are two related but different techniques used for elemental analysis in various fields.
The main difference between the two techniques is the way they measure the elemental composition of a sample. ICP-OES uses a high-resolution spectrometer to measure the wavelengths of light emitted by the sample, whereas ICP-AES uses filters or prisms to select specific wavelengths of light for measurement.
In terms of cost, an ICP machine can range from tens of thousands to hundreds of thousands of dollars, depending on its features, complexity, and brand.
Industries that use atomic absorption spectroscopy include but are not limited to: environmental science, geology and mining, pharmaceuticals, food and beverage, agriculture, and forensic science. Atomic absorption spectroscopy can be used to detect and quantify various elements present in a sample, including heavy metals, nutrients, and trace elements. For example, in environmental science, it is used to monitor the levels of pollutants in soil and water. In food and beverage industries, it can be used to ensure the safety and quality of products by detecting the presence of harmful contaminants or nutritional elements. In geology and mining, it is used to identify and quantify minerals in ores and rocks.
In simpler terms, ICP-OES and ICP-AES are two methods of analyzing the elements present in a sample. ICP-OES uses a spectrometer, and ICP-AES uses filters or prisms. An ICP machine is expensive and is used in various industries such as environmental science, mining, and food and beverage to detect the presence of various elements in a sample.
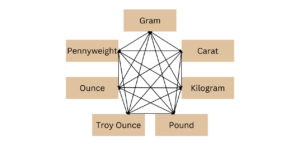
PRECIOUS METALS UNITS OF WEIGHT CONVERSION CALCULATOR
Karat, gram, pennyweight, ounce, troy ounce, pound, kilogram

Gold Plated Parts Calculator
Calculating the gold content of plated parts.
To get first estimation of gold content, it is possible to use the following calculator:
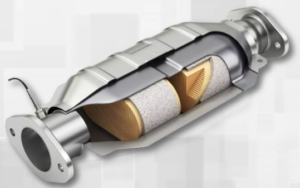
Car Catalytic Converter Value Calculator
This calculator, designed to calculate the catalytic converters precious metals value, based on precious metals assay of the monolith.