LOOKING TO INVEST IN GOLD OR SILVER LOOK UP GOLDBROKR IS A GREAT OPRTIUNTY DONT MISS IT !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
Understanding Auto Catalytic Converters TWCC, FWCC, DOC, SCR, NOx, TRAP, and DPF
Dealing with auto catalytic converters, you probably have a good understanding about the precious metal content that creates its value as scrap.
In this article we will cover different mechanisms of auto catalytic converters, which will broaden your knowledge and understanding of the catalyst mechanism.
TWCC (TWC)
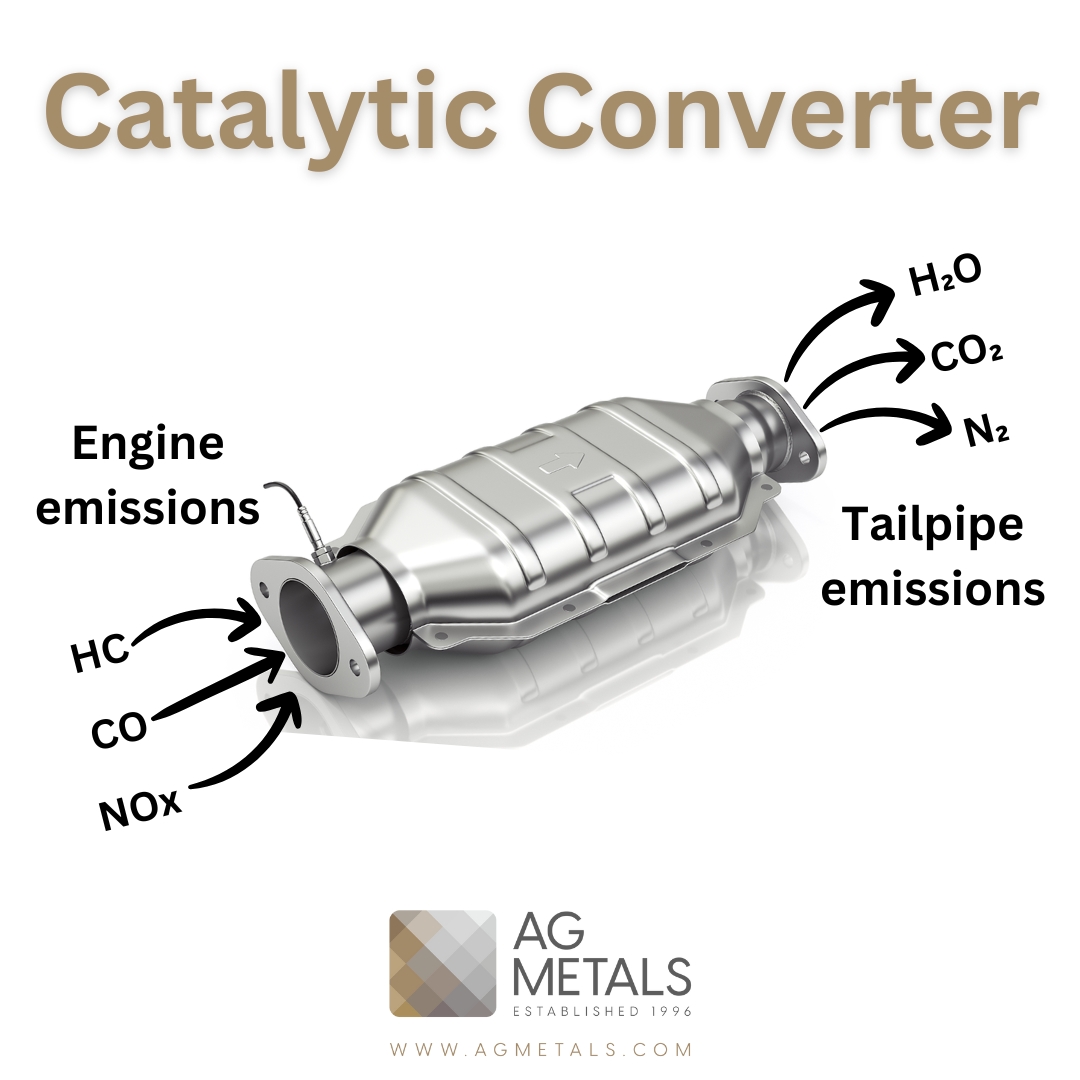
A three-way catalytic converter (TWC) is a device used in the exhaust system of gasoline-powered vehicles to reduce the emission of harmful pollutants. It is called a “three-way” converter because it simultaneously reduces three types of pollutants – nitrogen oxides (NOx), carbon monoxide (CO), and hydrocarbons (HC) – into less harmful gases such as nitrogen, carbon dioxide, and water vapor.
The TWC contains a ceramic honeycomb-like structure coated with a thin layer of catalyst material, usually a combination of precious metals such as platinum, palladium, and rhodium. When the engine runs, the exhaust gases pass through the TWC, where the catalyst triggers a chemical reaction that breaks down the harmful pollutants into less harmful ones.
The process occurs in three stages. In the first stage, the catalyst oxidizes carbon monoxide and hydrocarbons into carbon dioxide and water vapor. In the second stage, the catalyst reduces nitrogen oxides into nitrogen gas and oxygen. Finally, in the third stage, any remaining oxygen in the exhaust gas reacts with carbon monoxide and hydrocarbons to produce carbon dioxide and water vapor.
Overall, the TWC plays an essential role in reducing harmful emissions from gasoline-powered vehicles, helping to mitigate air pollution and its adverse effects on public health and the environment.
FWCC (FWC)
A four-way catalytic converter is a device used in the exhaust system of diesel-powered vehicles to reduce the emission of harmful pollutants. It is called a “four-way” converter because it simultaneously reduces four types of pollutants – nitrogen oxides (NOx), carbon monoxide (CO), hydrocarbons (HC), and particulate matter – into less harmful gases such as nitrogen, carbon dioxide, and water vapor.
The four-way catalytic converter is similar to the three-way catalytic converter used in gasoline-powered vehicles, but it also includes an additional system for reducing particulate matter emissions. The particulate matter reduction system typically includes a diesel particulate filter, which captures and stores the particulate matter emissions from the engine. The filter then periodically burns off the stored particulate matter, converting it into harmless gases.
In the four-way catalytic converter, the catalyst material is typically a combination of platinum, palladium, and rhodium. The converter has a honeycomb-like structure that provides a large surface area for the catalyst to react with the exhaust gases.
The reduction of NOx in a four-way catalytic converter occurs through a process called selective catalytic reduction (SCR), which involves the use of a reducing agent, such as ammonia or urea, to convert NOx into nitrogen gas and water vapor. This process occurs at high temperatures and requires careful management of the air-to-fuel ratio to ensure that the right amount of reducing agent is supplied.
The reduction of CO and HC in a four-way catalytic converter is similar to that in a three-way converter. The catalyst material triggers chemical reactions that break down these pollutants into less harmful gases, such as carbon dioxide, water vapor, and nitrogen gas.
Overall, the four-way catalytic converter plays an essential role in reducing harmful emissions from diesel-powered vehicles, helping to mitigate air pollution and its adverse effects on public health and the environment.
DOC
A Diesel Oxidation Catalyst (DOC) is a type of catalytic converter that is used to reduce the emission of harmful pollutants from the exhaust of diesel-powered engines. It is typically installed in the exhaust system of diesel vehicles, including trucks, buses, and heavy-duty equipment.
The Diesel Oxidation Catalyst works by oxidizing and converting the pollutants, such as carbon monoxide (CO) and hydrocarbons (HC), into less harmful gases, such as carbon dioxide (CO2) and water vapor (H2O). The catalyst material in a DOC is typically a precious metal, such as platinum or palladium, which is coated onto a ceramic honeycomb structure. The honeycomb structure provides a large surface area for the catalyst to interact with the exhaust gases.
When the exhaust gases pass through the Diesel Oxidation Catalyst, the catalyst promotes chemical reactions that convert the harmful pollutants into less harmful gases. Specifically, the CO is oxidized to CO2, while the HC is converted into H2O and CO2. In addition, the DOC can also reduce the emission of particulate matter, which are tiny solid or liquid particles that are suspended in the exhaust.
The Diesel Oxidation Catalyst is often used as the first stage of a multi-stage emission control system in diesel engines. The subsequent stages can include a diesel particulate filter (DPF) and/or a selective catalytic reduction (SCR) system, which further reduce the emission of harmful pollutants.
Overall, the Diesel Oxidation Catalyst is an important component of the exhaust system in diesel engines, helping to reduce the emission of harmful pollutants and improve air quality.
SCR
Selective Catalytic Reduction (SCR) is a technology used in the exhaust systems of diesel engines to reduce the emission of harmful pollutants, particularly nitrogen oxides (NOx). SCR systems work by injecting a reducing agent, usually an aqueous solution of urea, into the exhaust stream. The reducing agent reacts with the NOx in the presence of a catalyst to convert the pollutants into nitrogen, water vapor, and small amounts of carbon dioxide.
The SCR process occurs in a catalytic converter, which is typically located downstream of the diesel oxidation catalyst (DOC) and the diesel particulate filter (DPF) in a multi-stage emission control system. The SCR catalyst is typically made of a metal, such as platinum or rhodium, which is coated onto a honeycomb-like structure.
The reducing agent, which is stored in a separate tank on the vehicle, is metered into the exhaust stream based on the engine’s operating conditions. When the reducing agent enters the hot exhaust stream, it decomposes into ammonia (NH3) and carbon dioxide (CO2). The ammonia then reacts with the NOx on the SCR catalyst, reducing it to nitrogen gas (N2) and water vapor (H2O).
The SCR process is highly efficient, with typical NOx conversion rates of over 90%. It also has the advantage of being able to operate over a wide range of engine conditions and exhaust temperatures, making it suitable for use in a variety of diesel engines and applications.
Overall, selective catalytic reduction is a proven technology for reducing the emission of harmful pollutants from diesel engines, helping to improve air quality and reduce the negative impacts of diesel exhaust on human health and the environment.
NOx TRAP
Nitrogen Oxides (NOx) Trap, also known as NOx Storage and Reduction (NSR), is a technology used in the exhaust systems of gasoline and diesel-powered vehicles to reduce the emission of NOx pollutants. NOx trap systems work by capturing NOx in a special catalyst material, and then periodically releasing and reducing the captured NOx to less harmful nitrogen gas (N2) and water vapor (H2O).
NOx traps are typically made up of a catalytic coating, such as barium oxide, and a support material, such as alumina. The catalyst material captures the NOx from the engine exhaust, while the support material provides a large surface area for the catalyst to interact with the exhaust gases.
When the NOx trap becomes saturated with NOx, the engine management system reduces the air-to-fuel ratio, causing the exhaust gases to become rich in hydrocarbons (HC) and carbon monoxide (CO). This “rich” exhaust gas then flows through the NOx trap, where the trapped NOx reacts with the HC and CO to form ammonia (NH3) and carbon dioxide (CO2).
The ammonia then reacts with the remaining NOx on the catalyst material, reducing it to nitrogen gas and water vapor. This process is called the “regeneration” phase of the NOx trap cycle. Once the NOx trap is fully regenerated, the engine management system returns to its normal operating mode, and the cycle begins again.
NOx traps are effective at reducing NOx emissions under light load and low-temperature conditions. However, they have limited effectiveness at high engine loads and temperatures, which can lead to thermal deactivation of the catalyst material. Additionally, NOx trap systems require a relatively high fuel consumption to regenerate the trap, which can impact fuel economy.
Overall, NOx trap technology provides a useful method for reducing NOx emissions from gasoline and diesel-powered vehicles, especially under low-load and low-temperature conditions. However, it may not be suitable for all engine applications and operating conditions, and alternative NOx reduction technologies, such as selective catalytic reduction (SCR), may be required to meet more stringent emission regulations.
DPF
A Diesel Particulate Filter (DPF) is a device that is installed in the exhaust system of diesel engines to reduce the emission of harmful particulate matter or soot. DPFs work by trapping and collecting these particulate emissions, and then periodically burning them off to regenerate the filter.
The DPF is typically made up of a ceramic or metallic substrate, which is coated with a catalyst material such as platinum or palladium. The substrate is designed to trap the particulate emissions while allowing the exhaust gases to flow through.
As the exhaust gases pass through the DPF, the particulate matter or soot is captured on the surface of the substrate. Over time, this soot buildup can lead to increased backpressure in the exhaust system, reducing engine performance and fuel efficiency.
To prevent this buildup, the DPF is designed to periodically regenerate the filter by burning off the trapped particulate matter. This is typically done through a process called “passive regeneration”, where the filter is regenerated automatically during normal driving conditions. During passive regeneration, the exhaust gas temperatures are increased to a level where the accumulated soot is burned off, converting it into ash.
If passive regeneration is not sufficient to clean the filter, the engine management system will initiate an active regeneration cycle. During an active regeneration, the engine injects additional fuel into the exhaust system, raising the exhaust gas temperature and causing the soot to burn off.
DPFs are effective at reducing particulate emissions from diesel engines, with typical particulate matter reduction rates of 80-90%. However, they do require periodic regeneration to maintain their effectiveness, which can impact fuel economy and increase maintenance costs. Additionally, DPFs may not be suitable for all engine applications and operating conditions, and alternative particulate matter reduction technologies, such as selective catalytic reduction (SCR), may be required to meet more stringent emission regulations.
Catalytic converters in hybrid cars
Catalytic converters in hybrid cars function similarly to those in conventional gasoline vehicles but are adapted to the specific needs of hybrids, which combine internal combustion engines with electric motors. Here’s a breakdown of how they work and some points specific to hybrids:
Function: The primary function of a catalytic converter in any vehicle, including hybrids, is to reduce harmful emissions from the exhaust system. It converts toxic gases and pollutants like nitrogen oxides, carbon monoxide, and hydrocarbons into less harmful substances such as nitrogen, carbon dioxide, and water vapor through chemical reactions.
Adaptation to Hybrids: In hybrid vehicles, the catalytic converter must handle a different operational pattern due to the intermittent use of the internal combustion engine (ICE). The ICE in a hybrid car might not always be running, especially in city driving where the electric motor often takes over to reduce fuel consumption and emissions.
Temperature Challenges: For a catalytic converter to effectively reduce emissions, it needs to reach a certain operating temperature. In hybrid vehicles, maintaining this temperature can be a challenge because the ICE shuts off periodically (e.g., during idle or low-speed conditions when the electric motor is used). Manufacturers may use strategies like closer placement of the catalytic converter to the engine or using electric heating elements to ensure it remains effective.
Durability and Efficiency: Hybrid cars tend to require catalytic converters that are both durable and efficient due to the variable temperatures and operational modes. The stop-start nature of the engine in hybrids means that the converter must quickly reactivate each time the engine restarts.
Emissions Standards: Catalytic converters in hybrids are designed to meet stringent emissions standards. As hybrids emit less CO2 due to better fuel efficiency, their catalytic converters are optimized to reduce other pollutants that are still significant due to the combustion process.
Overall, the design and operation of catalytic converters in hybrid vehicles are critical to achieving the environmental benefits that hybrids are known for, while facing unique challenges due to their dual-engine systems.
The Valuable Core of Scrap Catalytic Converters: A Deep Dive into Precious Metals
In the evolving world of automotive recycling, the catalytic converter stands out due to its content of precious metals like platinum, palladium, and rhodium. These metals are key to the converter’s function of reducing vehicle emissions, but they also make scrap catalytic converters highly valuable in the recycling market.
Each week, in our newsletter “Catalyst of the Week” and “Tip of the Week”, we delve into the intricacies of various types of catalytic converters, focusing on their precious metal content and the factors influencing their scrap value. This understanding is crucial not only for recyclers but also for automotive professionals and environmental specialists who seek to optimize resource use and recovery.
Precious Metals in Focus
Platinum: Used in catalytic converters due to its stability and effectiveness in chemical reactions necessary for reducing toxic emissions. It is the main catalyst in diesel engines’ converters.
Palladium: More commonly used in gasoline engine converters, palladium serves a similar purpose to platinum but has seen a significant price increase due to its efficiency and growing demand in the market.
Rhodium: The most expensive of the three, rhodium is prized for its superior ability to break down nitrous oxides, a major pollutant emitted by vehicles.
These metals are embedded in the ceramic structure of the converter’s monolith, which is then coated onto a honeycomb structure to maximize the surface area for reactions.
Market Dynamics
The value of scrap catalytic converters fluctuates daily, influenced by several factors:
- Precious Metal Prices: As global market prices for platinum, palladium, and rhodium vary, so does the value of scrap converters. These metals are traded commodities, and their prices are impacted by economic policies, mining output, and industrial demand.
- Currency Fluctuations: Exchange rates also play a critical role, as metal prices are often pegged to the dollar. Changes in currency values can significantly affect converter scrap prices internationally.
- Catalyst Catalog Number: Each catalytic converter model has a unique catalog number, which helps determine its specific composition and, consequently, its scrap value. Many apps and resources are available to help recyclers and traders identify these numbers and assess value accurately.
Tools for Valuation
To assist in the valuation process, numerous apps and databases have been developed, allowing users to input a catalytic converter’s catalog number and instantly receive up-to-date pricing based on current metal values. These tools are indispensable for recyclers who need to make quick decisions in a highly dynamic market.
In summary, the scrap value of catalytic converters is a complex interplay of market dynamics, precious metal content, and technological tools. Through our “Catalyst of the Week” and “Tip of the Week,” we aim to provide our readers with detailed, actionable information that empowers them to navigate this lucrative yet fluctuating landscape effectively. By understanding these elements, stakeholders can optimize their operations and contribute to a more sustainable automotive future.
NOTES:
If you are interested in auto catalytic scrap you may look on our newsletter for valuble information.
If you need calculator for Auto catalytic scrap value prees calculator