How To Avoid XRF Assay Mistakes When Assaying Precious Metals
Introduction
In this article, we will address a critical issue – avoiding XRF (X-ray Fluorescence) assay mistakes when determining the composition of precious metals. These mistakes can lead to misguided financial decisions and a sense of frustration, and we are here to guide you through the intricacies of the process.
The Role of Handheld XRF Devices
Handheld XRF devices have gained popularity due to their convenience and quick results. However, it’s essential to comprehend their limitations and operational mechanisms to ensure accurate assessments of precious metal content. One common pitfall occurs when using the “Precious Metals” mode on these devices, which can yield inaccurate results, falsely indicating the presence of platinum, gold, palladium, or iridium in your sample.
Understanding XRF Assays
Before delving into practical examples, let’s briefly explain why these errors can occur. XRF assays rely on the energy emitted by atoms in the sample, creating a unique spectrum with energy peaks for each element present. The “Precious Metals” mode narrows the element range to enhance precision. However, this narrowing can lead to software misinterpretation, especially for elements with similar energy peaks.
Common Misinterpretations
To illustrate these issues, we’ll examine three real-life examples:
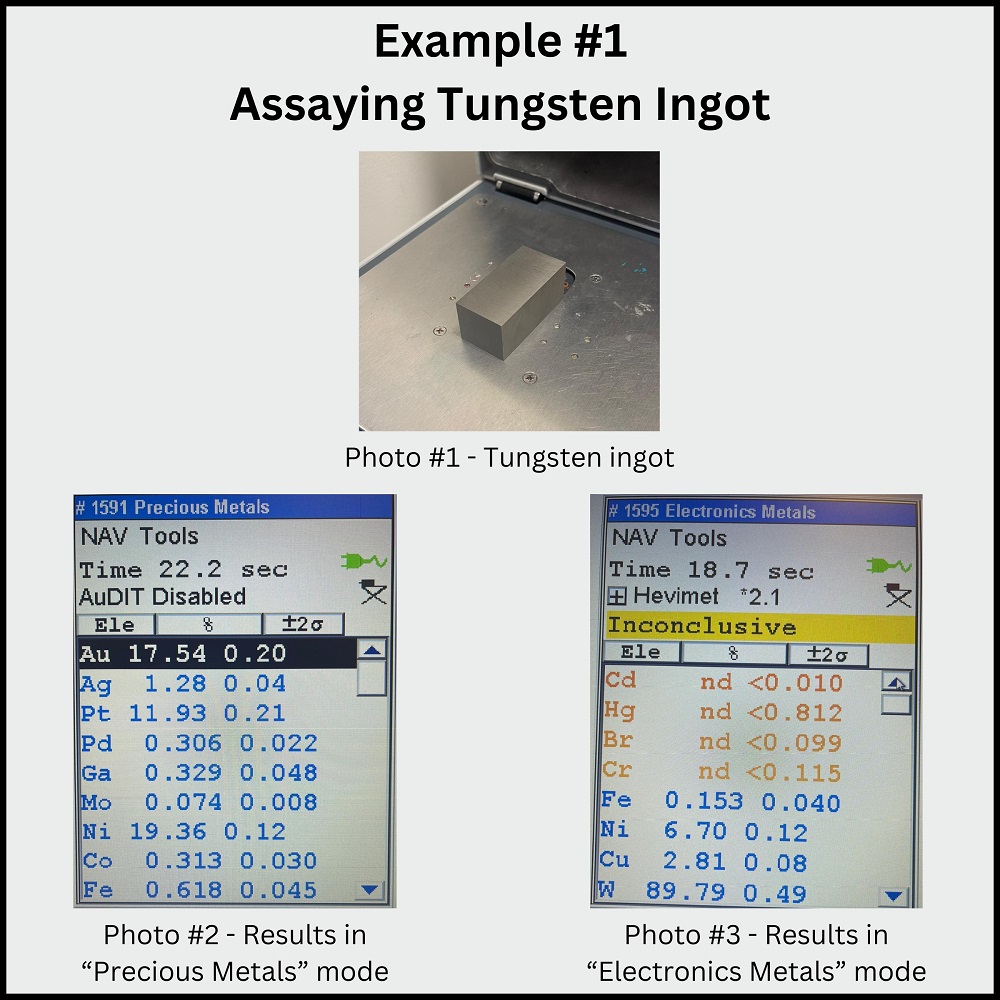
Example #1 (images 1-3 below): Tungsten Ingot. When assaying a 90% tungsten ingot in “Precious Metal” mode, the results may falsely suggest a high gold and platinum content. However, switching to the “Electronics Metals” mode yields accurate results, indicating no precious metal content.
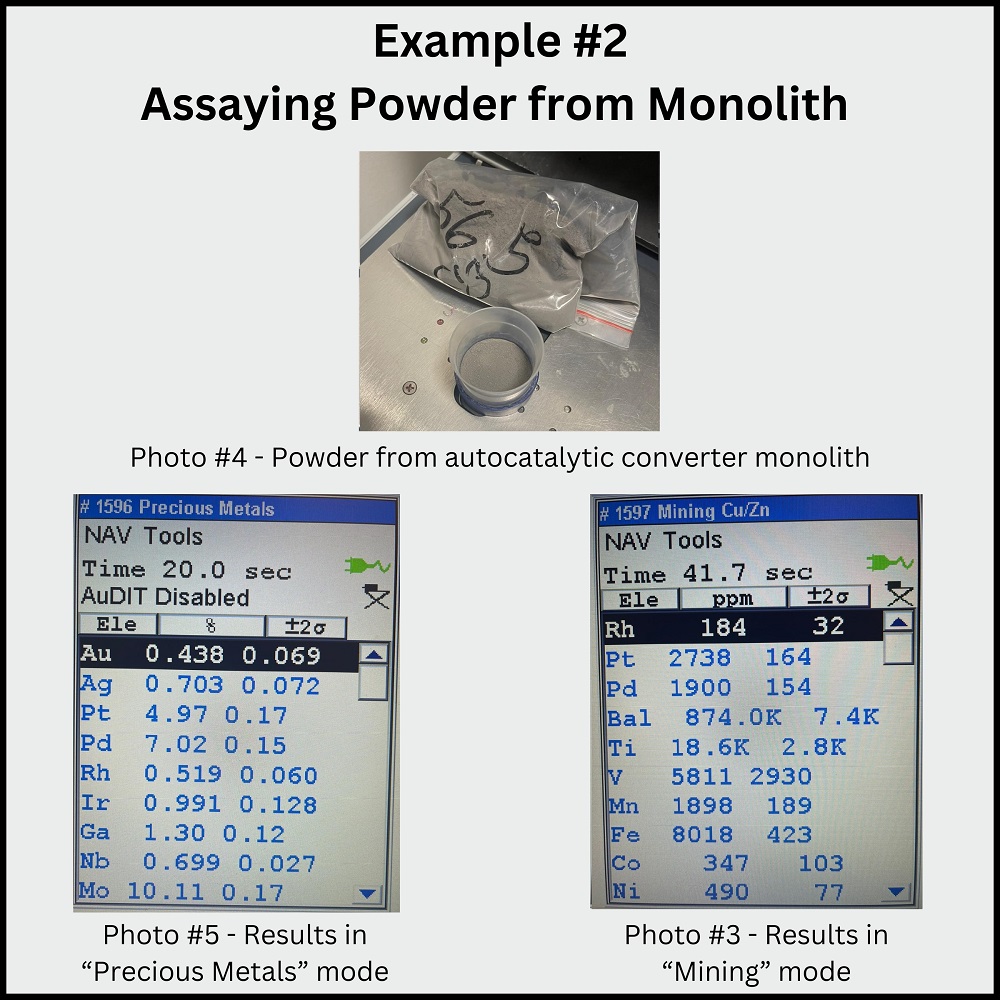
Example #2 (images 4-6 below): Catalytic Converter Powder Assaying powder from an auto catalytic converter monolith using the “Precious Metal” mode may result in unrealistic values for gold, platinum, and palladium content. Switching to the “Mining Mode” provides more accurate results, measured in parts per million (PPM).
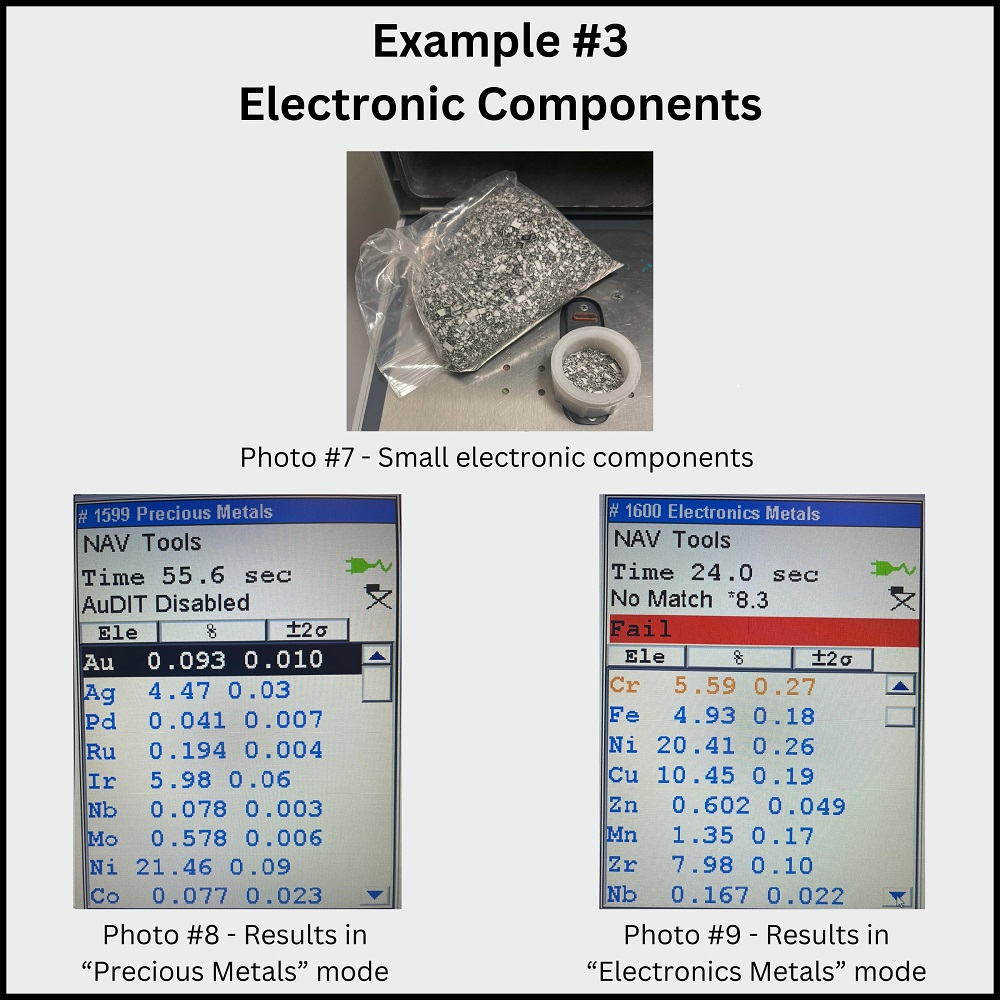
Example #3 (images 7-9 below): Electronic Components Assaying small electronic components in the “Precious Metals” mode may lead to unrealistic readings, such as high gold and iridium content. However, the “Electronics Metals” mode offers vastly different results, with silver and palladium present.
Conclusion
- XRF assay requires more than just pressing a button. It demands a solid understanding of its modes and their implications.
- Always critically assess your results, ensuring they align with logical expectations.
- It’s advisable to conduct assays using multiple modes, starting with a broader element range to reduce the likelihood of misinterpretation.
- Note that different handheld XRF devices may have varying mode names, but the core principles remain consistent.
- Samples containing oxides or humidity, such as powders, will not yield accurate results, as XRF cannot detect hydrogen or oxygen.
- As demonstrated in example #3, avoid assaying components directly; they must be processed to a homogenate stage before analysis.
- Understanding the XRF spectrum and its energy peaks is crucial for accurate precious metal assessments. Consider studying this aspect for more in-depth insights.
We hope this article helps you avoid XRF assay mistakes, enabling you to make informed decisions when dealing with precious metals. For more valuable content, don’t forget to follow A.G. Metals on Facebook and LinkedIn.
