The Main Differences Between Melting and Smelting
Although the two terms melting and smelting sound similar, they are different terms that have different applications. Melting is the process of liquefying a solid substance by heating. It is the process by which a substance changes from the solid phase to the liquid phase. Smelting is the process by which a metal is obtained at temperatures beyond the melting point from its ore. Both processes involve heating a substance to a higher temperature. The main difference between melting and smelting is that melting converts a solid substance into a liquid whereas smelting converts an ore to its purest form.
Smelting is done from basic metals like iron, copper, and silver where chemicals, such as reducing agents, are used to wash out another element from ore into gas or slag.
Another way to look at it is that melting is a spontaneous process as opposed to smelting which is a non-spontaneous process. Melting is mainly done to mold an element or substance into a particular shape to use in various ways, whereas smelting is done for extracting metals like copper, iron, silver, and gold.
A good example for small recyclers: when recycling palladium and silver from Pd-Ag capacitors, as a first step we ball-mill it into a powder and then smelt it with fluxes (chemicals such as reducing agents). Results demonstrate that from 1,000 grams of Ag-Pd capacitor powder you can usually get a 130 gram metal ingot of all the metal components of the powder (Ni, Cu, Bi, Ag, Pd). The balance is composed of slag (waste).
Smelting Process Examples:
Iron Smelting:
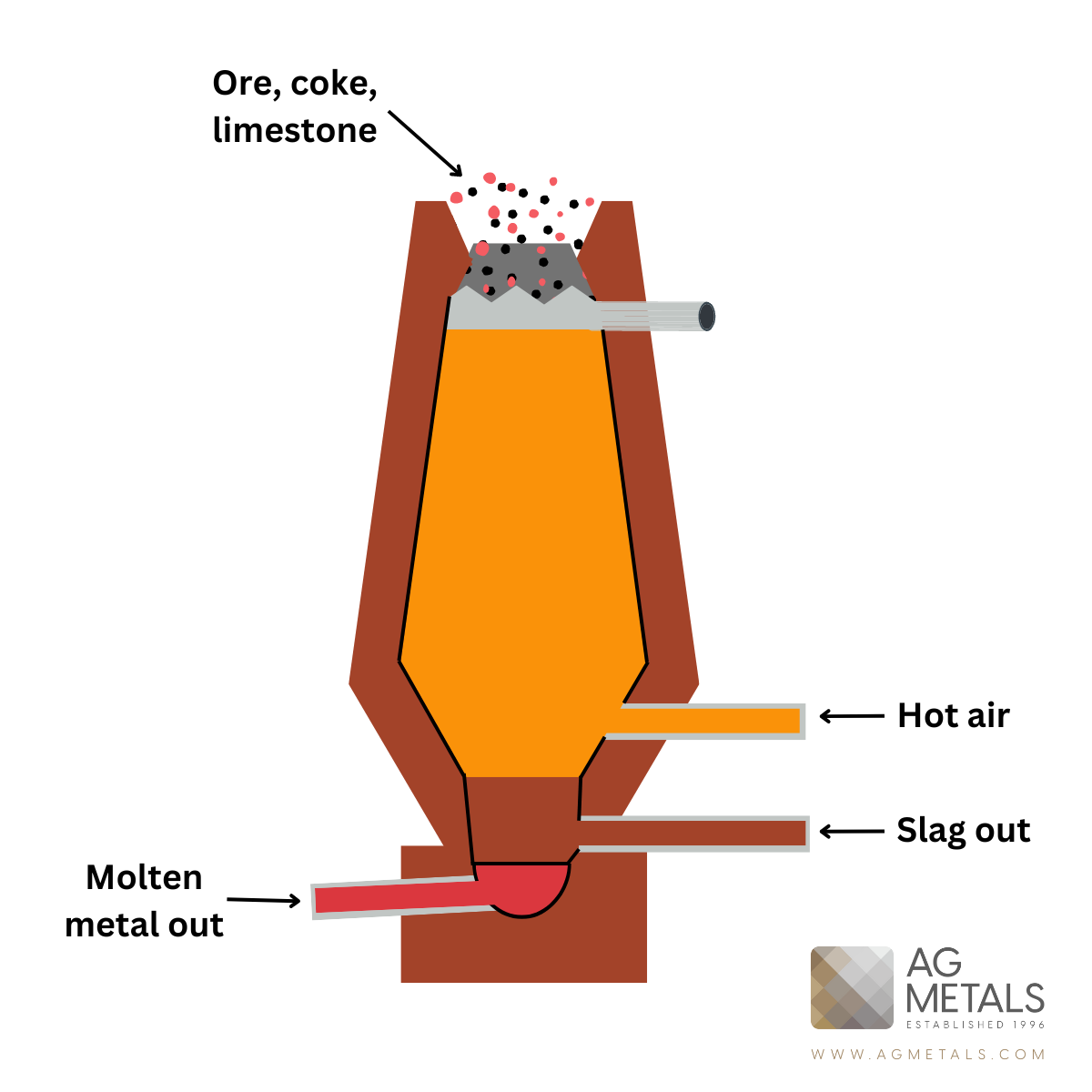
- Raw Material: Iron ore (typically hematite or magnetite), coke (carbonized coal), and limestone.
- Process: The iron ore is heated in a blast furnace along with coke and limestone. The coke provides the heat and reduces the iron ore to molten iron, while limestone helps remove impurities. The molten iron, along with slag, is tapped from the bottom of the furnace.
Copper Smelting:
- Raw Material: Copper ore (usually chalcopyrite), flux (such as silica), and a reducing agent (often coke or charcoal).
- Process: The copper ore is heated in a smelter, where the chemical reactions take place. The flux helps remove impurities, and the reducing agent causes the copper to separate from the ore and become molten. The molten copper is then cast into desired shapes.
Melting Process Examples:
Melting Gold:
- Raw Material: Gold in the form of jewelry, coins, or other gold items.
- Process: The gold items are placed in a crucible and heated to a high temperature, typically using a torch or a furnace. Gold has a relatively low melting point (around 1,948 degrees Fahrenheit or 1,064 degrees Celsius), so it melts easily. Once melted, the molten gold can be poured into molds to create new shapes or objects.
Melting Silver:
- Raw Material: Silver in the form of silverware, coins, or other silver items.
- Process: Similar to melting gold, the silver items are placed in a crucible and heated to the melting point of silver (around 1,763 degrees Fahrenheit or 961 degrees Celsius). The heat causes the silver to melt into a liquid state. The molten silver can then be cast into new forms or used for various applications, such as creating silver bars or jewelry.
These examples demonstrate the basic process of melting precious metals like gold and silver for various applications, including recycling and crafting new items.